
 |
|
| Home | Vorinostat | CI-994 | MS-275 | BML-210 | M344 | NVP-LAQ824 | Panobinostat | Mocetinostat | PXD101 | LBH-589LBH-589 is a hydroxamic acid based HDACi with a structure similar to vorinostat. It is in phase I clinical trials for cutaneous T-cell lymphoma as an oral agent. It has a longer half-life than vorinostat. 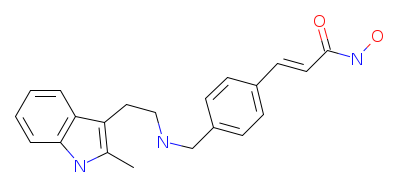
IUPAC Name: In vitro Panobinostat induces cell cycle arrest and apoptosis through both caspase dependent and caspase independent pathways in various tumor cell types at nanomolar concentrations. In vivo LBH-589 inhibits tumor angiogenesis as evidenced by blocking new blood vessel formation in human prostate carcinoma cell PC 3 xenografts. References: |
| CBHA | PCI-24781 | ITF2357 | Valproic Acid | Trichostatin A | Sodium Butyrate | |
|
© 2010-2019 www.hdacis.com |